SKY WORLD NEWS / In 1957, the pharmaceutical company Chemie Grünenthal introduced a new sedative, thalidomide, to the market. Sold mainly in Germany under the name of Contergan and in Great Britain under that of Distaval, thalidomide was presented as a substitute for barbiturates.
These, used since the beginning of the century, had acquired a bad reputation because of their side effects, and in particular their toxicity in the event of overdose.
In comparison, thalidomide was so safe that it was impossible to kill yourself with it, even swallowing an entire box of pills.
This safety aspect and the anti-nausea effects of thalidomide led Chemie Grünenthal to promote its use in pregnant women in a dozen countries, under different names.
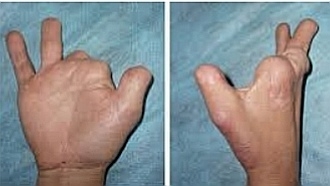
In Canada, thalidomide was released in late 1959 under the name Kevadon. Unfortunately, what was not known at the time was that exposure of the fetus to thalidomide between the fifth and seventh weeks of pregnancy could cause serious malformations.
About 20 percent of children whose mothers used thalidomide were born with limbs that were atrophied or even completely absent.
How is it possible that a supposedly safe drug, as the advertisement opposite indicates, could have caused such a tragedy? Thalidomide had been tested extensively before it was marketed.
Clinical trials, in humans and several lines of laboratory animals, did not reveal any toxicity. Opponents of animal testing are using the thalidomide case to support their view that animal testing is not a good predictor of toxicity in humans.
What supporters reply that on the contrary, it reinforces the need for more tests.
None of the tests performed by Chemie Grünenthal were directly related to the possibility of teratogenic effects.
In fact, at the time, the fetus was believed to be protected by the placental barrier and toxicity testing in pregnant females was not required. Another lesson from the tragedy is the realization that different species can react very differently to the same substance.
Thalidomide does not have a teratogenic effect in the rodent species that Chemie Grünenthal had used, but affects many other species, especially primates, which are close to us. Today thethe pharmacovigilance rules require the examination of these possibilities in laboratory animals.
Two human aspects associated with the thalidomide case are worth mentioning. The first concerns Dr. Frances Kelsey, a McGill University graduate who, more than any other, saved the thalidomide tragedy in the United States.
Dr Kelsey, who worked for the FDA, was responsible for approving new drugs. While other countries, based on studies provided by the company, had quickly approved thalidomide, Dr. Kelsey had not. She had blocked the file after learning that some patients were reporting tingling fingers.
Wondering if this might be a sign of more serious problems, she had decided, despite pressure from the company, to get more information before acting.
It was good for him, for in the meantime Dr. William McBride, the other person intimately linked to the history of thalidomide, had made a name for himself.
In a letter to the medical journal The Lancet, the Australian obstetrician reported the emergence of serious birth defects in his practice.
He was the first to link these deformities to pregnant women who had taken thalidomide.
This letter alerted the medical community around the world and brought to light the scale of the tragedy.
Thalidomide was withdrawn from the market in 1961 after causing severe birth defects in more than 10,000 children in nearly 50 countries.
Doctors Kelsey and McBride both became heroes for their action. But, in the case of Dr. McBride, another aspect of his career is less brilliant.
Following his experience with thalidomide, he decided to investigate the possibility that other drugs could also have teratogenic effects. In 1981, he published a study indicating that a drug sold under the name Bendectin (Dicletin in Canada) was responsible for malformations in laboratory animals.
A study that shocked the scientific community and, in particular, its co-authors (for good reasons, in the case of the latter).
Doctor McBride had not consulted them before including their names in the article. If that had been the case, they would not have agreed to be included, as it turned out that Dr. McBride had manipulated the results to implicate Bendectin as a teratogen.
Following this case, a disciplinary committee struck Dr. McBride off the Australian medical register. The teratogenic effect of thalidomide comes from its ability to inhibit angiogenesis, the growth of blood vessels.
Angiogenesis is also the process necessary for the development of certain cancers. As a result, ironically today, thalidomide, a molecule that caused one of the greatest medical tragedies, is being used, in a framed way of course, to prolong the lives of thousands of patients around the world.